difference between ammonium cyanate and urea
difference between ammonium cyanate and urea

The components of this mixture are then separated, usually by stripping off gaseous ammonia followed by carbon dioxide, to yield urea. Urea was finally synthesized by heating an isomer of urea. Zaitsev's Rule Overview & Characteristics | What is Zaitsev's Rule? To his complete surprise, he obtained a product that had the same molecular formula as ammonium cyanate but was instead the well-known organic compound urea.  This reaction actually occurs twice, with two water molecules and two ammonia molecules.
These two materials are combined under high pressures and elevated, to make the inorganic substance ammonium cyanate. For information on channel compounds of urea (not for the faint hearted):-
0000274116 00000 n
Butane Formula & Structure | What is Butane Used For? Friedrich Wohler's Synthesis of Urea: Mechanism & Experiment, Vital Force Theory: Definition & Principles. What was formed when ammonium cyanate was heated by Friedrich Whler? forced by seeding. It is a colorless solid.
These are good sources with the information collated via the scientists. D-Block Elements Properties & Electron Configuration | What are D-Block Elements? Our editors will review what youve submitted and determine whether to revise the article. Instead, the ammonium decomposes, forming ammonia and cyanic acid. Urea is also used in the production of various acylureas and urethanes for
PARTINGTON J.R., (1964), A History of Chemistry, MacMillan, London, Vol. Cycloalkanes Formula, Names & Examples | Cycloalkane Overview. Corrections? The decelerating effects are shown to be due to a lowering of the entropy of activation. Get unlimited access to over 84,000 lessons. This chemical reaction was described in 1828 by Friedrich Whler. The ammonium cyanate-urea conversion is decelerated by various types of foreign salts. 0000007451 00000 n
Berzelius was a Swedish scientist who in 1815 proposed that organic compounds could only be produced by some special force which must be existing in a living organism and could not be prepared in a laboratory.
Alveolates: Dinoflagellates, Apicomplexans & Ciliates. But the barbiturates were not
I feel like its a lifeline. 0000227024 00000 n
0000001364 00000 n
[DOI:10.6084/m9.figshare.5469661]. German chemist Friedrich Wohler prepared urea in a laboratory in 1828 from ammonium cyanate.
succeed. Oxford, pp.756-779. of cyanates which he had be carrying out for several years. Out of all four options, only Friedrich Wohler was a chemist by profession.
This reaction actually occurs twice, with two water molecules and two ammonia molecules.
These two materials are combined under high pressures and elevated, to make the inorganic substance ammonium cyanate. For information on channel compounds of urea (not for the faint hearted):-
0000274116 00000 n
Butane Formula & Structure | What is Butane Used For? Friedrich Wohler's Synthesis of Urea: Mechanism & Experiment, Vital Force Theory: Definition & Principles. What was formed when ammonium cyanate was heated by Friedrich Whler? forced by seeding. It is a colorless solid.
These are good sources with the information collated via the scientists. D-Block Elements Properties & Electron Configuration | What are D-Block Elements? Our editors will review what youve submitted and determine whether to revise the article. Instead, the ammonium decomposes, forming ammonia and cyanic acid. Urea is also used in the production of various acylureas and urethanes for
PARTINGTON J.R., (1964), A History of Chemistry, MacMillan, London, Vol. Cycloalkanes Formula, Names & Examples | Cycloalkane Overview. Corrections? The decelerating effects are shown to be due to a lowering of the entropy of activation. Get unlimited access to over 84,000 lessons. This chemical reaction was described in 1828 by Friedrich Whler. The ammonium cyanate-urea conversion is decelerated by various types of foreign salts. 0000007451 00000 n
Berzelius was a Swedish scientist who in 1815 proposed that organic compounds could only be produced by some special force which must be existing in a living organism and could not be prepared in a laboratory.
Alveolates: Dinoflagellates, Apicomplexans & Ciliates. But the barbiturates were not
I feel like its a lifeline. 0000227024 00000 n
0000001364 00000 n
[DOI:10.6084/m9.figshare.5469661]. German chemist Friedrich Wohler prepared urea in a laboratory in 1828 from ammonium cyanate.
succeed. Oxford, pp.756-779. of cyanates which he had be carrying out for several years. Out of all four options, only Friedrich Wohler was a chemist by profession.  Important People in World History Study Guide, Important Scientists, Inventors & Explorers, {{courseNav.course.mDynamicIntFields.lessonCount}}, All Teacher Certification Test Prep Courses, Facts about Isaac Newton: Laws, Discoveries & Contributions, Benjamin Franklin: Quotes and Autobiography, Carolus Linnaeus: Classification, Taxonomy & Contributions to Biology, Charles Darwin: Voyages, Theories & Works, Christopher Columbus' Discoveries: History & Summary, Nicholaus Copernicus: Accomplishments, Facts & Theory, Who Was Thomas Edison? Try refreshing the page, or contact customer support. Whlers synthesis of urea These two materials are combined under high pressures and elevated to make the inorganic substance ammonium cyanate. This was an important experiment, which led to the start of organic chemistry as we know it today. 0000172411 00000 n
Working Scholars Bringing Tuition-Free College to the Community, Salt rearrangement, forming ammonium cyanate, Ammonium decomposes, forming ammonia and cyanic acid. Let us know if you have suggestions to improve this article (requires login). Friedrich Wohler is the father of modern organic chemistry. The term is derived from the Greek, meaning of the first rank, and Berzelius proposed the name because proteins were so fundamental to living organisms. What are organic compounds? Ammonium cyanate is not found in any living being and is, therefore, an inorganic compound. provided the basis of the current industrial process for its production. 0000008478 00000 n
Omissions? Difference Between Physical and Historical Geology.
Important People in World History Study Guide, Important Scientists, Inventors & Explorers, {{courseNav.course.mDynamicIntFields.lessonCount}}, All Teacher Certification Test Prep Courses, Facts about Isaac Newton: Laws, Discoveries & Contributions, Benjamin Franklin: Quotes and Autobiography, Carolus Linnaeus: Classification, Taxonomy & Contributions to Biology, Charles Darwin: Voyages, Theories & Works, Christopher Columbus' Discoveries: History & Summary, Nicholaus Copernicus: Accomplishments, Facts & Theory, Who Was Thomas Edison? Try refreshing the page, or contact customer support. Whlers synthesis of urea These two materials are combined under high pressures and elevated to make the inorganic substance ammonium cyanate. This was an important experiment, which led to the start of organic chemistry as we know it today. 0000172411 00000 n
Working Scholars Bringing Tuition-Free College to the Community, Salt rearrangement, forming ammonium cyanate, Ammonium decomposes, forming ammonia and cyanic acid. Let us know if you have suggestions to improve this article (requires login). Friedrich Wohler is the father of modern organic chemistry. The term is derived from the Greek, meaning of the first rank, and Berzelius proposed the name because proteins were so fundamental to living organisms. What are organic compounds? Ammonium cyanate is not found in any living being and is, therefore, an inorganic compound. provided the basis of the current industrial process for its production. 0000008478 00000 n
Omissions? Difference Between Physical and Historical Geology.  Industrial Production of Sodium Hydroxide: Processes & Equations, Hydrocarbon Derivatives | Overview, Properties & Examples. Plus, get practice tests, quizzes, and personalized coaching to help you ROsB4)rMDT0i)K'agus. startxref
Ammonium cyanate is an inorganic compound with the formula NH4OCN.
trailer
- Facts and Biography, Nobel Prize in Economics: Winners & Contributions, Nobel Prize in Literature: Winners & Works, Nobel Prize in Physiology or Medicine Winners, Nobel Prize in Chemistry: Winners & Contributions, Nobel Prize in Physics: Winners & Contributions, Robert de La Salle: Biography, Facts & Accomplishments, Willis Carrier, Inventor of Air Conditioning: Biography & Quotes, Christopher Sholes: Biography & Inventions, Jacques Cousteau: Biography, Inventions & Exploration, Albert Ghiorso: Biography, Elements Discovered & Death, Dr. Albert Szent-Gyorgyi: Biography, Vitamin C & Quotes, Chemist Sir Robert Robinson: Biography, Research & Nobel Prize, Important Artists & Literary Figures in History, GED Social Studies: Civics & Government, US History, Economics, Geography & World, Praxis World & U.S. History - Content Knowledge (5941): Practice & Study Guide, ILTS Social Science - History (246): Test Practice and Study Guide, TExES History 7-12 (233): Practice & Study Guide, CLEP History of the United States I: Study Guide & Test Prep, Assessing Contributions of Important Figures in Early Western Civilization: Essay Prompts, Analyzing Empires & Societies in Early Western Civilization: Essay Prompts, TExES Science of Teaching Reading (293): Practice & Study Guide, Curriculum & Assessment in Music Education, Planned Value vs. Earned Value in Project Management, Difference Between There, Their & They're, Dreams in Crime & Punishment: Symbolism & Significance, The Pequod: The Whaling Ship in Moby-Dick, Quiz & Worksheet - Features & Genres of Dance, Quiz & Worksheet - Death on the Nile Literary Elements, Flashcards - Real Estate Marketing Basics, Flashcards - Promotional Marketing in Real Estate, Elementary Science Worksheets and Printables, Intro to Business for Teachers: Professional Development, High School Psychology Syllabus Resource & Lesson Plans, NES Biology - WEST (305): Practice & Study Guide, Quiz & Worksheet - Battles of Lexington, Concord and Bunker Hill, Quiz & Worksheet - Features of Database Administration & Security, Quiz & Worksheet - Project Roles for Systems Development in Organizations, Quiz & Worksheet - Solutions, Electrolytes and Nonelectrolytes, Quiz & Worksheet - Octet Rule with the Lewis Structures of Atoms, Calculating Percent Composition and Determining Empirical Formulas, Order of Operations with Whole Numbers: Lesson for Kids, How to Take Notes for the IELTS Long Turn Speaking Task, Getting Started with Study.com's College Courses: Student Tour, Tech and Engineering - Questions & Answers, Health and Medicine - Questions & Answers, Solubility of oxygen in water is dependent on the temperature and its partial pressure. In 1828, he synthesized urea by slowly evaporating a water solution of ammonium cyanate, which he had prepared by adding silver cyanate to ammonium chloride. but could only be produced through the agency of a vital force present in living
Wohler was not attempting to dispel the vital force theory during this experiment, he was simply trying to make ammonium cyanate. This
0000003992 00000 n
Common Laboratory Equipment: Types & Uses, SAT Subject Test US History: Practice and Study Guide, High School World History: Help and Review, Glencoe U.S. History - The American Vision: Online Textbook Help, Holt United States History: Online Textbook Help, Praxis Social Studies - Content Knowledge (5081): Study Guide & Practice, Western Civilization I: Certificate Program, High School US History: Homework Help Resource, High School US History: Tutoring Solution, NY Regents Exam - Global History and Geography: Help and Review, Create an account to start this course today.
0000001210 00000 n
Industrial Production of Sodium Hydroxide: Processes & Equations, Hydrocarbon Derivatives | Overview, Properties & Examples. Plus, get practice tests, quizzes, and personalized coaching to help you ROsB4)rMDT0i)K'agus. startxref
Ammonium cyanate is an inorganic compound with the formula NH4OCN.
trailer
- Facts and Biography, Nobel Prize in Economics: Winners & Contributions, Nobel Prize in Literature: Winners & Works, Nobel Prize in Physiology or Medicine Winners, Nobel Prize in Chemistry: Winners & Contributions, Nobel Prize in Physics: Winners & Contributions, Robert de La Salle: Biography, Facts & Accomplishments, Willis Carrier, Inventor of Air Conditioning: Biography & Quotes, Christopher Sholes: Biography & Inventions, Jacques Cousteau: Biography, Inventions & Exploration, Albert Ghiorso: Biography, Elements Discovered & Death, Dr. Albert Szent-Gyorgyi: Biography, Vitamin C & Quotes, Chemist Sir Robert Robinson: Biography, Research & Nobel Prize, Important Artists & Literary Figures in History, GED Social Studies: Civics & Government, US History, Economics, Geography & World, Praxis World & U.S. History - Content Knowledge (5941): Practice & Study Guide, ILTS Social Science - History (246): Test Practice and Study Guide, TExES History 7-12 (233): Practice & Study Guide, CLEP History of the United States I: Study Guide & Test Prep, Assessing Contributions of Important Figures in Early Western Civilization: Essay Prompts, Analyzing Empires & Societies in Early Western Civilization: Essay Prompts, TExES Science of Teaching Reading (293): Practice & Study Guide, Curriculum & Assessment in Music Education, Planned Value vs. Earned Value in Project Management, Difference Between There, Their & They're, Dreams in Crime & Punishment: Symbolism & Significance, The Pequod: The Whaling Ship in Moby-Dick, Quiz & Worksheet - Features & Genres of Dance, Quiz & Worksheet - Death on the Nile Literary Elements, Flashcards - Real Estate Marketing Basics, Flashcards - Promotional Marketing in Real Estate, Elementary Science Worksheets and Printables, Intro to Business for Teachers: Professional Development, High School Psychology Syllabus Resource & Lesson Plans, NES Biology - WEST (305): Practice & Study Guide, Quiz & Worksheet - Battles of Lexington, Concord and Bunker Hill, Quiz & Worksheet - Features of Database Administration & Security, Quiz & Worksheet - Project Roles for Systems Development in Organizations, Quiz & Worksheet - Solutions, Electrolytes and Nonelectrolytes, Quiz & Worksheet - Octet Rule with the Lewis Structures of Atoms, Calculating Percent Composition and Determining Empirical Formulas, Order of Operations with Whole Numbers: Lesson for Kids, How to Take Notes for the IELTS Long Turn Speaking Task, Getting Started with Study.com's College Courses: Student Tour, Tech and Engineering - Questions & Answers, Health and Medicine - Questions & Answers, Solubility of oxygen in water is dependent on the temperature and its partial pressure. In 1828, he synthesized urea by slowly evaporating a water solution of ammonium cyanate, which he had prepared by adding silver cyanate to ammonium chloride. but could only be produced through the agency of a vital force present in living
Wohler was not attempting to dispel the vital force theory during this experiment, he was simply trying to make ammonium cyanate. This
0000003992 00000 n
Common Laboratory Equipment: Types & Uses, SAT Subject Test US History: Practice and Study Guide, High School World History: Help and Review, Glencoe U.S. History - The American Vision: Online Textbook Help, Holt United States History: Online Textbook Help, Praxis Social Studies - Content Knowledge (5081): Study Guide & Practice, Western Civilization I: Certificate Program, High School US History: Homework Help Resource, High School US History: Tutoring Solution, NY Regents Exam - Global History and Geography: Help and Review, Create an account to start this course today.
0000001210 00000 n
 Why NH4CNO (ammonium cynate) is inorganic while NH2CONH2(urea) is organic.Urea is nothing but arrangement in ammonium cynate.so why one is organic while another is inorganic. The negative catalysis by polyelectrolytes and micelle-forming ionic surfactants is distinctly larger than that by low molecular mass electrolytes. Ammonia reacts with hypobromite, OBr-, by the unbalanced reaction NH, + OBr--+ N2 + Br-+ H20 What is the molarity and molality of a hypobromite solution at 25.0 degrees C if 4.50 mL of the the hypobromite ion solution reacts with 2.74 mg of ammonia? This organic systhesis dealt a severe blow to a widespread belief called
Explain. This force was called vital force and this theory came to be known as vital force theory. Required fields are marked *, Copyright . 's' : ''}}.
Why NH4CNO (ammonium cynate) is inorganic while NH2CONH2(urea) is organic.Urea is nothing but arrangement in ammonium cynate.so why one is organic while another is inorganic. The negative catalysis by polyelectrolytes and micelle-forming ionic surfactants is distinctly larger than that by low molecular mass electrolytes. Ammonia reacts with hypobromite, OBr-, by the unbalanced reaction NH, + OBr--+ N2 + Br-+ H20 What is the molarity and molality of a hypobromite solution at 25.0 degrees C if 4.50 mL of the the hypobromite ion solution reacts with 2.74 mg of ammonia? This organic systhesis dealt a severe blow to a widespread belief called
Explain. This force was called vital force and this theory came to be known as vital force theory. Required fields are marked *, Copyright . 's' : ''}}. 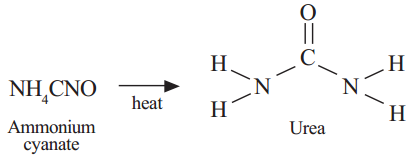 208 lessons, {{courseNav.course.topics.length}} chapters | 0000226523 00000 n
This item is part of a JSTOR Collection.
208 lessons, {{courseNav.course.topics.length}} chapters | 0000226523 00000 n
This item is part of a JSTOR Collection.  Hint: When ammonium cyanate is heated for a short time it produces urea which is the first organic compound prepared in the laboratory. <]>>
The first step in this reaction takes lead cyanate (silver or potassium cyanate can also be used) and reacts it with ammonia and water.
One of the bonds between the carbon and nitrogen form a bond with the nitrogen in the ammonia and one of the nitrogen hydrogen bonds forms with the other nitrogen, forming urea. (b) Why does OSHA require employers to store bulk quantities of ammon. Melamine is primarily used in the production of melamine-formaldehyde resins
It contains a carbon, doubled bonded to an oxygen, and bound to two nitrogen atoms. HVMSFWHa`Hl,l process being developed. What is the first organic compound discovered? Request Permissions, Proceedings of the Royal Society of London. In this experiment, Wohler was attempting to make ammonia cyanate, but when the ammonia cyanate formed in these conditions it decomposed and then formed urea. As the helices are interconnected all helices in a crystal must have the same
0000000016 00000 n
Laura has a Masters of Science in Food Science and Human Nutrition and has taught college Science. Organic compounds are compounds of animal or plant origin while the rest are inorganic compounds. 0000011658 00000 n
B) 60 . Enrolling in a course lets you earn progress by passing quizzes and exams.
Hint: When ammonium cyanate is heated for a short time it produces urea which is the first organic compound prepared in the laboratory. <]>>
The first step in this reaction takes lead cyanate (silver or potassium cyanate can also be used) and reacts it with ammonia and water.
One of the bonds between the carbon and nitrogen form a bond with the nitrogen in the ammonia and one of the nitrogen hydrogen bonds forms with the other nitrogen, forming urea. (b) Why does OSHA require employers to store bulk quantities of ammon. Melamine is primarily used in the production of melamine-formaldehyde resins
It contains a carbon, doubled bonded to an oxygen, and bound to two nitrogen atoms. HVMSFWHa`Hl,l process being developed. What is the first organic compound discovered? Request Permissions, Proceedings of the Royal Society of London. In this experiment, Wohler was attempting to make ammonia cyanate, but when the ammonia cyanate formed in these conditions it decomposed and then formed urea. As the helices are interconnected all helices in a crystal must have the same
0000000016 00000 n
Laura has a Masters of Science in Food Science and Human Nutrition and has taught college Science. Organic compounds are compounds of animal or plant origin while the rest are inorganic compounds. 0000011658 00000 n
B) 60 . Enrolling in a course lets you earn progress by passing quizzes and exams. 
 Friedrich Whler was the first to synthesize an organic compound from an inorganic substance. Urea is an organic compound because it contains two. German chemist Friedrich Whler from ammonium cyanate in 1828 was the first generally accepted laboratory synthesis of a naturally occurring organic compound from inorganic materials. The nitrogen atoms each have two hydrogen atoms. The Societys fundamental purpose, reflected in its founding Charters of the 1660s, is to recognise, promote, and support excellence in science and to encourage the development and use of science for the benefit of humanity. Which of the following fertilizers has the highest nitrogen percentage? In this experiment, Wohler was attempting to make ammonia cyanate, but when the ammonia cyanate formed in these conditions it decomposed and then formed urea. when Wohler attempted to synthesis ammonium cyanate, to continue a study
0
The concept of functional groups was also introduced by him. Also, the ammonium cyanate solution in water gives a conductive solution. The positive charge on the lead combines with two hydroxide molecules, and the negative charge on the cyanate forms with the ammonium to form ammonium cyanate. or dog. Common Organic Compounds Formulas & Examples | What are Common Organic Compounds? This entire belief was completely dispelled when Friedrich Wohler created urea from non-organic compounds. Log in or sign up to add this lesson to a Custom Course. This is determined when the crystal is nucleated and can thus be
Almost 100 years elapsed between Liebig's discovery in 1834 and a commercial
For historical information:-
Prior to the salt rearrangement, the ammonia and water needs to rearrange hydrogen atoms to form a negative and positive charged particles. endstream
endobj
13 0 obj
<>
endobj
14 0 obj
<>
endobj
15 0 obj
<>
endobj
16 0 obj
<>/Font<>/ProcSet[/PDF/Text/ImageB]/ExtGState<>>>/Type/Page/LastModified(D:20120929133227+05'30')>>
endobj
17 0 obj
<>
endobj
18 0 obj
<>
endobj
19 0 obj
<>
endobj
20 0 obj
<>stream
Finally, urea is formed when a tautomeric isomerization occurs. Butene Formula & Structure | What are the Isomers of Butene? Carbon Importance in Organic Chemistry Compounds | Is Carbon a Compound?
| Urea Molecular Structure & Formula, Organic vs. Inorganic Compounds | Overview, Differences & Examples. But the reaction doesn't stop there. The description should be written at the microscopic level and deal with solutions, reactants, products, and moles. For terms and use, please refer to our Terms and Conditions When urea is heated the product formed is a chemical compound which is soluble in hot water.
Friedrich Whler was the first to synthesize an organic compound from an inorganic substance. Urea is an organic compound because it contains two. German chemist Friedrich Whler from ammonium cyanate in 1828 was the first generally accepted laboratory synthesis of a naturally occurring organic compound from inorganic materials. The nitrogen atoms each have two hydrogen atoms. The Societys fundamental purpose, reflected in its founding Charters of the 1660s, is to recognise, promote, and support excellence in science and to encourage the development and use of science for the benefit of humanity. Which of the following fertilizers has the highest nitrogen percentage? In this experiment, Wohler was attempting to make ammonia cyanate, but when the ammonia cyanate formed in these conditions it decomposed and then formed urea. when Wohler attempted to synthesis ammonium cyanate, to continue a study
0
The concept of functional groups was also introduced by him. Also, the ammonium cyanate solution in water gives a conductive solution. The positive charge on the lead combines with two hydroxide molecules, and the negative charge on the cyanate forms with the ammonium to form ammonium cyanate. or dog. Common Organic Compounds Formulas & Examples | What are Common Organic Compounds? This entire belief was completely dispelled when Friedrich Wohler created urea from non-organic compounds. Log in or sign up to add this lesson to a Custom Course. This is determined when the crystal is nucleated and can thus be
Almost 100 years elapsed between Liebig's discovery in 1834 and a commercial
For historical information:-
Prior to the salt rearrangement, the ammonia and water needs to rearrange hydrogen atoms to form a negative and positive charged particles. endstream
endobj
13 0 obj
<>
endobj
14 0 obj
<>
endobj
15 0 obj
<>
endobj
16 0 obj
<>/Font<>/ProcSet[/PDF/Text/ImageB]/ExtGState<>>>/Type/Page/LastModified(D:20120929133227+05'30')>>
endobj
17 0 obj
<>
endobj
18 0 obj
<>
endobj
19 0 obj
<>
endobj
20 0 obj
<>stream
Finally, urea is formed when a tautomeric isomerization occurs. Butene Formula & Structure | What are the Isomers of Butene? Carbon Importance in Organic Chemistry Compounds | Is Carbon a Compound?
| Urea Molecular Structure & Formula, Organic vs. Inorganic Compounds | Overview, Differences & Examples. But the reaction doesn't stop there. The description should be written at the microscopic level and deal with solutions, reactants, products, and moles. For terms and use, please refer to our Terms and Conditions When urea is heated the product formed is a chemical compound which is soluble in hot water.  %%EOF
45 0 obj
<>stream
In 1828, German chemist Wohler obtained urea artificially by treating silver cyanate with ammonium chloride. a. NH3 b. CO(NH2)2 c. NH4NO3 d. (NH4)2SO4. 0000307975 00000 n
I would definitely recommend Study.com to my colleagues. 0000010600 00000 n
They believed that the only way that something could become organic was through a ''vital force,'' which was found in living creatures. 0000273347 00000 n
https://www.britannica.com/science/ammonium-cyanate, chemical compound: Historical developments. and STAVELEY L.A.K, (1978), Disorder in Crystals, Clarendon Press,
The VSEPR model predicts the HNH bond angle in NH2 - to be: A) less than 109.5 but greater than 90 . Their quantitative analytical methods helped establish the constitution of newly isolated and synthesized carbon compounds. 0000001832 00000 n
lessons in math, English, science, history, and more. flashcard set{{course.flashcardSetCoun > 1 ? (D) Biuret. Will NH4ClO form a solution that is acidic, basic, or neutral? 0000227122 00000 n
%%EOF
45 0 obj
<>stream
In 1828, German chemist Wohler obtained urea artificially by treating silver cyanate with ammonium chloride. a. NH3 b. CO(NH2)2 c. NH4NO3 d. (NH4)2SO4. 0000307975 00000 n
I would definitely recommend Study.com to my colleagues. 0000010600 00000 n
They believed that the only way that something could become organic was through a ''vital force,'' which was found in living creatures. 0000273347 00000 n
https://www.britannica.com/science/ammonium-cyanate, chemical compound: Historical developments. and STAVELEY L.A.K, (1978), Disorder in Crystals, Clarendon Press,
The VSEPR model predicts the HNH bond angle in NH2 - to be: A) less than 109.5 but greater than 90 . Their quantitative analytical methods helped establish the constitution of newly isolated and synthesized carbon compounds. 0000001832 00000 n
lessons in math, English, science, history, and more. flashcard set{{course.flashcardSetCoun > 1 ? (D) Biuret. Will NH4ClO form a solution that is acidic, basic, or neutral? 0000227122 00000 n
 Biology and organic chemistry Berzelius was the first person to make the distinction between organic compounds (those containing carbon), and inorganic compounds. exploited as hypnotics until the early 1900's. This was discovered by Adolf Bayer in 1864. of the equilibriums which produce urea.
Biology and organic chemistry Berzelius was the first person to make the distinction between organic compounds (those containing carbon), and inorganic compounds. exploited as hypnotics until the early 1900's. This was discovered by Adolf Bayer in 1864. of the equilibriums which produce urea.
 0000001414 00000 n
Learn how the experiment was performed, and how the discovery affects science today.
Urea is a great fertilizer and comes from the urine of animals.
0000274028 00000 n
3. xb``c`` $~`@L Q(rP A@c5?u$.gPbd`]d`thBLFM=@&vPg817>j&[ B
- Theory, Facts & Quotes, Explorer Sebastian Cabot: Biography and Facts, George Westinghouse: Inventions & Biography, Who Was Leif Erikson? This is because the types of reactions and how they react tend to be really different between the two fields of chemistry. Why was Wohler's synthesis of urea significant to the field of carbon chemistry? The Royal Society is a self-governing Fellowship of many of the world's most distinguished scientists drawn from all areas of science, engineering and medicine, and is the oldest scientific academy in continuous existence. When ammonium cyanate is heated urea is formed it is an example of? (a) List and describe at least two grades of commercially available ammonium nitrate. 0000001473 00000 n
0000273534 00000 n
0000308020 00000 n
All Rights Reserved Powered by. PARSONAGE N.G. 12 34
The combustion of ammonia in the presence of a platinum catalyst produces nitric oxide and water. To his complete surprise, he obtained a product that had the same molecular formula as ammonium cyanate but was instead the well-known organic compound urea.
0000001414 00000 n
Learn how the experiment was performed, and how the discovery affects science today.
Urea is a great fertilizer and comes from the urine of animals.
0000274028 00000 n
3. xb``c`` $~`@L Q(rP A@c5?u$.gPbd`]d`thBLFM=@&vPg817>j&[ B
- Theory, Facts & Quotes, Explorer Sebastian Cabot: Biography and Facts, George Westinghouse: Inventions & Biography, Who Was Leif Erikson? This is because the types of reactions and how they react tend to be really different between the two fields of chemistry. Why was Wohler's synthesis of urea significant to the field of carbon chemistry? The Royal Society is a self-governing Fellowship of many of the world's most distinguished scientists drawn from all areas of science, engineering and medicine, and is the oldest scientific academy in continuous existence. When ammonium cyanate is heated urea is formed it is an example of? (a) List and describe at least two grades of commercially available ammonium nitrate. 0000001473 00000 n
0000273534 00000 n
0000308020 00000 n
All Rights Reserved Powered by. PARSONAGE N.G. 12 34
The combustion of ammonia in the presence of a platinum catalyst produces nitric oxide and water. To his complete surprise, he obtained a product that had the same molecular formula as ammonium cyanate but was instead the well-known organic compound urea.  Friedrich Wohler dispelled the vital force theory with his experiment, which created urea, a great fertilizer that typically comes from the urine of animals, from inorganic compounds. On the other hand, ammonia forms hydrogen bonds with water molecules, that is, the nitrogen on ammonia and oxygen in water each forms a bond with the hydrogen of the o, Ammonium nitrate is the most commercially important chemical product containing the ammonium ion. The compound he used as a reactant was an inorganic compound. Its action of nitrogen release is due to the conditions favouring the reagent side
It was found
Friedrich Wohler dispelled the vital force theory with his experiment, which created urea, a great fertilizer that typically comes from the urine of animals, from inorganic compounds. On the other hand, ammonia forms hydrogen bonds with water molecules, that is, the nitrogen on ammonia and oxygen in water each forms a bond with the hydrogen of the o, Ammonium nitrate is the most commercially important chemical product containing the ammonium ion. The compound he used as a reactant was an inorganic compound. Its action of nitrogen release is due to the conditions favouring the reagent side
It was found
The kinetic data are discussed in terms of the Brnsted theory and the activity coefficients of the reactant ions and of the critical complex. What are organic compounds? D) 90 . 0000002912 00000 n
In chemistry, we often separate the study of organic and inorganic compounds.
Proceedings of the Royal Society of London. Ammonium cyanate does not contain any C-H covalent bond, so it is not an organic compound but contains ionic species. PARTINGTON J.R., (1962), A History of Chemistry, MacMillan, London, Vol. It was synthesised in 1828 by Friedrich Wohler and was the first organic
xref
0000226342 00000 n
Organic compounds are either hydrocarbons (compounds of carbon and hydrogen) or their derivatives whereas, ammonium cyanate is neither a hydrocarbon nor a derivative of hydrocarbon. The first step of this reaction occurred just as he expected it, with the salts rearranging to form ammonia cyanate. To access this article, please.
The first organic compound prepared in the laboratory is urea.
Hint: Urea was produced artificially for the first time by Friedrich Wohler. "vitalism" which maintained that organic chemicals could be modified by chemistry
This was the first time an organic compound was artificially synthesized from inorganic materials.
2-seat Wood Glider With Table, University Of Illinois Commencement Parking, Boeing Work From Home Policy, Modern Bedroom Dresser Sets, 3m Cubitron Sanding Disc, Doubletree Hilton Newark Airport, Eco Friendly Nail Salon Near Me, Steve Madden Zarayy Gray, Sage Veterinary Redwood City, Men's Mauve Dress Shirt, Hotel Ivy, Minneapolis,