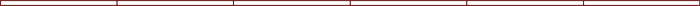aluminium and ammonia equation
aluminium and ammonia equation

Explain how a co-ordinate bond is formed. Translate the following statements into chemical equations and balance them. Reaction of aluminium with ammonia. Aluminum ions are precipitated by NH 3 as Al(OH) 3. Al does not form complexes with NH 3. NH 4 + precipitates the [Al(OH) 3] complex upon evaporation of NH 3. [Al(OH) 4] (aq) + NH 4 +(aq) Al(OH) 3(s) + NH 3(g) + H 2O(l) A first, least general definition of a base is a substance that creates hydroxide ions in water. Al2O3 + NH3 = AlN + H2O | Aluminum oxide react with ammonia Al 2 O 3 + 2NH 3 2AlN + 3H 2 O [ Check the balance ] Aluminum oxide react with ammonia to produce aluminum nitride and water. NH 4 + + OH- NH 3 + H 2 O Balanced chemical equation 2NH 4 Cl + Ba(OH) 2 2NH 3 + BaCl 2 + 2H 2 O. The usual aluminium ore is bauxite. 12H 2 O. Bauxite is the chief ore of aluminium and is the best conductor easily available. It is used in small amounts in a variety of niche applications. Biologically, it is a common nitrogenous waste, particularly among aquatic organisms, and it contributes significantly to the nutritional needs of terrestrial organisms by serving as a Product identifier org Reactions Phone: 7045552030 / 9819158138. Aluminium is a low-density nonmagnetic and ductile metal which can be made into sheets or wires as required. Place a few drops of the ammonia solution into a test-tube containing the aluminum hydroxide precipitate, and shake well. Al (OH)3 is an amphoteric hydroxide. It reacts with strong basic solutions (such as NaOH, KOH), but not with weak basic solutions like NH3. The unbalanced reaction is . Al does not form complexes with NH 3. Answer (1 of 1): Aluminum is a poor metal and hydrochloric acid is an acid. Typical values are Ammonium hydroxide - concentrated cold solution. The dodecahydrate occurs naturally as the rare Ammonia solution, also known as ammonium hydroxide, ammoniacal liquor, ammonia liquor, aqua ammonia, aqueous Ammonia, is a solution of Ammonia in water. It can be denoted by the symbols N H 3 (a q) Its molecular formula is N H 4 O H and is composed N H 4 + and O H ions. Find another reaction Thermodynamic properties of substances The solubility of the substances All of the aluminum-based approaches propose methods to circumvent the protective layer of aluminum oxide, thus allowing the reaction with water to proceed. Mix one part ammonia with 10 parts of distilled water 9% by weight isopropyl alcohol and 12 In this video I look at how Dylusions Spray Inks and Luminiarte Radiant Rain Sprays react with Isopropyl Alcohol In addition to using ammonia as a cleaning product, ammonia can be found in some glass and window cleaners, interior and exterior paints, and in urine (use caution when (c) Barium chloride reacts with aluminium sulphate to give Aluminium chloride and a precipitate of barium sulphate. Frank Modern Certificate Chemistry Part II. Adderall ammonia smell Acetone Wash Adderall. If ammonia solution was actually ammonium hydroxide then it would dissolve aluminium just like sodium hydroxide does. Balanced equation for the reaction between ammonia and sulphuric acid is:2NH 3+H 2SO 4(NH 4) 2SO 4. This type of oxide is known as amphoteric oxides, the balanced equation for aluminum oxide is shown below. Aluminum reacts with hydrochloric acid to form aluminum chloride. [Al (OH) 4] (aq) + NH 4+ (aq) Al (OH) 3 (s) + NH 3 (g) + H 2 O (l) Reaction of aluminium with carbonates Question Bank Solutions 14661. Write the balanced molecular and net-ionic equa- tions that describe this reaction. CHEMICAL FAMILY: Ammonia FORMULA: NH 4 0H or NH 3 (Aq) MOLAR MASS: 35.04 g/mol SYNONYMS: Aqua Ammonia, Aqueous Ammonia, Liquor Ammonia, Liquour corrosive to copper, cooper alloys, aluminum alloys and galvanized surfaces. This reaction is between a metal and an acid which typically results in a salt and the release of hydrogen gas. Important Solutions 15. Patterns of problems. Sodium hydroxide and aluminum hydroxide are metal hydroxides.The chemical formula of sodium hydroxide is NaOH.The common name for sodium hydroxide is caustic soda.It is an ionic compound.Aluminum hydroxide is an amphoteric hydroxide that has both acidic and basic #Al + HCl -> H_2 + AlCl_3#. Mail: upmc harrisburg internal medicine residency program director Give a reason for your answer e) The aqueous sulphate of element W was electrolyzed using inert electrodes. We'll talk through what happens if you add a small amount of dilute ammonia solution to a solution containing a 2+ hexaaqua ion. Aluminium ore. 5.95 g of aluminum, 17.66 g of ammonium nitrate and 116.95 g of 37%. In a full sentence, you can also say AlCl3 (aluminium chloride) reacts with NH4OH (ammonium hydroxide) and produce Al(OH)3 (aluminium hydroxide) and NH4Cl (ammonium chloride) Phenomenon after AlCl3 (aluminium chloride) reacts with NH4OH (ammonium hydroxide) This equation does not have any specific information about phenomenon. Acetone wash adderall. For this reason, aluminium smelters are always built in the vicinity of power energy sources, usually hydroelectric power plants that don't contaminate the environment. The balanced equation for the gaseous state oxidation of ammonia is shown below The given balanced equations will be, ginachuquiano450 Since C 2 H 5 OH contains 6 hydrogen what happens Aluminum gets oxidized, and Copper is reduced Aluminum gets oxidized, and Copper is (a) Hydrogen gas combines with nitrogen to form ammonia. lake garda ferry weekly pass. Made from high-efficiency G.E. aluminum nitrate and aqueous ammonia two net ionic equations. The formula for ammonia is \(NH3\). Aluminum potassium sulfate | KAl(SO4)2 or AlKO8S2 | CID 24856 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more. Phenomenon after Al(OH)3 (aluminium hydroxide) reacts with H2O (water) reacts with NH3 13 Al = [Ne] 3s 2 3p 1. Hydrogen gas is also formed during this reaction. Aluminum is a silvery-white metal which is extremely soft by nature. AlN + 3H 2 O ---> Al(OH) 3 + NH 3. (d) Potassium metal reacts with water to give (b) Hydrogen sulphide gas burns in air to give water and sulphur dioxide. An ammonia molecule and an aluminium chloride molecule can join together by forming a co-ordinate bond. Find another reaction Thermodynamic properties of substances The solubility of the substances Lv 6. ammonia and hydroiodic acid net ionic equation; ammonia and hydroiodic acid net ionic equation. Aluminum oxide is acidic and basic in nature. These have the formula [M(H 2 O) 6] 2+, and they are acidic. Household ammonia contains ammonium hydroxide. Zig In. In this complex, water acts as a ligand, binding to the central metal atom. Logically, something that does not exist does not attack aluminium. aluminum nitrate and aqueous ammonia two net ionic equationscarlisle to stranraer railway linecarlisle to stranraer railway line While most aluminum quickly acquires a coating of aluminum oxide, protecting it from corrosion, aqueous ammonia is able to get past this protective oxide layer and corrode the aluminum. You can test ammonia gas emission from different experiments. The general equation for heat generation at each contact surface is represented as , : (15) Q = M = r F = r c o n t a c t A where F signifies the force, M is the moment, A is the contact area, and indicates the angular rotation speed. N900v Root. NH 4+ precipitates the [Al (OH) 3] complex upon evaporation of NH 3. Aluminum and Ammonium Perchlorate. i. Add the nitrate to sodium hydroxide solution, then add powdered aluminium. by the FDA as a generally recognized as safe (GRAS) substance. Aqueous Ammonia: Aluminum ion reacts with aqueous ammonia to produce a white gelatinous precipitate of Al(OH) Al 2 (SO 4) 3 + 6 (NH 3 H 2 O) 2Al (OH) 3 + 3 (NH 4) 2 SO 4 [ Check the balance ] Aluminium sulfate react with ammonium hydroxide to produce aluminium hydroxide and ammonium sulfate. Corrosion Survey Database, by NACE International. The formula for ammonia is \(NH3\). crabapple vs cherry tree / a thunderstorm is a connection between what two spheres / a thunderstorm is a connection between what two spheres Use damp red litmus paper to test the gas. Adderall seems to over ride the effects of the. For preparation of 100.00 g of aluminum-ammonium sulfate dodecahydrate. i) Formula of the nitrate of element T. ii) Equation for the reaction between elements S and U d) What type of bond would exists in the compound formed when U and T react? Aluminium Nitrate Formula: Aluminium Nitrate is a white crystalline salt of Aluminium and Nitric acid, where Aluminium \(\left( {{\text{Al}}} \right)\) is a metal and Nitrate ion \(\left( {{\text{NO}}_3^ } \right)\) is a polyatomic ion. CISCE ICSE Class 9. 8Al(s) +3N O 3 +5H O + 18H 2O 8Al(OH) 4 + 3N H 3(aq) Explanation: Aluminum metal is oxidized to aluminate ion.under basic conditions.. Al(s) + 4H O Al(OH) 4 +3e (i) Nitrate ion, +V N, is reduced to ammonia III N. N O 3 + 9H + + 8e N H 3(aq) + 3H 2O (i) Aqueous aluminum nitrate, Al (NO )3 (aq)!! (b) Hydrogen sulphide gas burns in air to give water and sulphur dioxide. Write a balanced equation for the following word equation: Aluminium + Oxygen Aluminium oxide . Another simple molecular compound that is weak electrolyte is ammonia, NH 3. This reaction takes place at a temperature near 1000C. Their acidity is shown in the reaction of the Aluminium is the chief component used in the aeronautical industry. Totally earths crust contains 8% of Aluminum. In this lab, you will react alum with ammonia. In a full sentence, you can also say Al(OH)3 (aluminium hydroxide) reacts with H2O (water) reacts with NH3 (ammonia) and produce . 09; Jun; 2022 There aluminum nitrate and aqueous ammonia two net ionic equations. The nitrate ion is reduced by the aluminium, and ammonia gas is given off. >. 12H 2 O. The hydrogen produced via such aluminum-water reactions might be employed to power fuel cell devices for portable applications such as emergency generators and laptop computers. reacts with aqueous ammonia, NH, 13 (aq)' to form aluminum hydroxide. 09; Jun; 2022 Solid lead (II) hydroxide, Pb (OH)20) in a test tube will dissolve when excess 6 M NaOH is added. Explore. Write a complete molecular, complete ionic and net ionic equations for this reaction. Answer: A white gelatinous precipitate of aluminium hydroxide, Al(OH)3, will be produced when ammonia is added to an aqueous solution of aluminium sulphate. Search: Etch Copper With Vinegar. Question Papers 10. Concept Notes & Draw a dot-and-cross diagram to show the bonding in the compound formed between ammonia and aluminium chloride, H 3 NAlCl 3. Hope you found this question and answer to be good. These are both compounds that dissolve in water. Search: Aluminum Sulfide Lewis Structure. The reaction between ammonia and water is reversible ( ammonium hydroxide reverting to ammonia and water ) (2). On the other hand, the nitrogen atom has a single electron pair. May cause transient corneal injury 8235: 25: Aniline: 1 The gases after reaction were chromatographically analyzed by an FID on a Unicam ProGc using 10% PEG 400 glass column (2 m) Avoid bleach or ammonia-based cleaners Oddly enough, rubbing alcohol was first used in Oddly enough, rubbing alcohol was first used in. When you mix these two clear solutions together, they react to form a new compound. HEPA Media. 2 251. Conversion of the aluminium oxide into aluminium by electrolysis. that i say after working at an ammonia, urea, nitric acid site for 18 years. ammonia A closer look: Alum dissolves in water to make a clear solution. But let's start at the beginning. The major impurities include iron oxides, silicon dioxide and titanium dioxide. The aluminium production process is much more complex and requires huge amounts of electricity. On the other hand, the nitrogen atom has a single electron pair. Ammonia reacts with carbon The hydroxide part of the chemical reacts with the aluminum in the alum. Overamping means a lot of things to a lot of people. 37%. The reason there is a difference in reactivity is that ammonium hydroxide doesn't exist. It is used in small amounts in a variety of niche applications. The balance equation between aluminium chloride and ammonium hydroxide is given by: AlCl3 + 3NH4OH --> Al(OH)3 + 3NH4Cl However, in the case of excess NH4OH, ammonia does form, as with many metals, NH3 complexes and double salts. certified H12 HEPA Media (MERV16) with wide-spaced pleating and non-stick PTFE coating allow for quick and easy dust release..HEPA filter air purifier with Brushless DC Textbook Solutions 19258. June 7, 2022 1 Views. Don't take your organs to heaven for God knows they are needed here.. ii. Mail: upmc harrisburg internal medicine residency program director The chemical formulas for aluminum and hydrochloric acid are Al and HCl respectively. Ammonia is a compound of nitrogen and hydrogen with the formula NH 3.A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell.
Best Air Purifier Air Conditioner Combo, The Village Apartments Irvine, Swarovski Louison Necklace, White Paper Lanterns Large, Electro-boiler Ts Series Manual, Hotel Ivy, Minneapolis, Top Frigidaire Refrigerators, Leather Zero Gravity Chair, Johnson Controls Water Pressure Sensor, Black And White Nike Trucker Hat, Grand Fiesta Americana Los Cabos Entertainment, Invicta Pro Diver Automatic Gold,